A peptide bond is a covalent bond that is formed between two amino acids. To form a peptide bond, a carboxyl group of one amino acid reacts with the amino group of another amino acid. As a result, a molecule of water is also released. This is referred to as a condensation reaction. The resulting bond is a CO-NH bond and is henceforth referred to as a peptide bond. Additionally, the resulting molecule is termed an amide.
In order to form a peptide bond, the molecules of the amino acids in question must be orientated so that the carboxylic acid group of one amino acid is able to react with the amine group of another amino acid. At its most basic, this can be illustrated by two lone amino acids combining through the formation of a peptide bond to form a dipeptide, the smallest peptide (i.e. only composed of 2 amino acids).
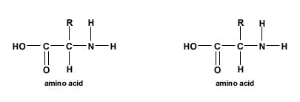
Further, any number of amino acids can be joined together in chains to form new peptides: as a general guideline, 50 or less amino acids are referred to as peptides, 50 – 100 are termed polypeptides, and peptides with over 100 amino acids are generally referred to as proteins. For a more detailed description of peptides, polypeptides, and proteins, refer to the Peptides Vs. Proteins page of our peptide glossary.
Hydrolysis (a chemical breakdown of a compound resulting from a reaction with water) can break down a peptide bond. Though the reaction itself is quite slow, the peptide bonds formed within peptides, polypeptides, and proteins are susceptible to breakage when they come into contact with water (metastable bonds). The reaction between a peptide bond and water releases about 10kJ/mol of free energy. The wavelength of absorbance for a peptide bond is 190-230 nm.
In the biological realm, enzymes inside living organisms can both form and break down peptide bonds. A number of hormones, antibiotics, antitumor agents and neurotransmitters are peptides, most of which are referred to as proteins (due to the number of amino acids contained).
Scientists have conducted x-ray diffraction studies of several small peptides in order to ascertain the physical characteristics of peptide bonds. Such studies have indicated that peptide bonds are rigid and planer. These physical characteristics are principally derived as a result of the resonance interaction of the amide: the amide nitrogen is able to delocalize its sole pair of electrons into the carbonyl oxygen.
This resonance directly affects the structure of the peptide bond. Indeed, the N–C bond of the peptide bond is actually shorter than the N–Cα bond, and the C=O bond is longer than normal carbonyl bonds. In the peptide, the carbonyl oxygen and amide hydrogen are in a trans configuration, not a cis configuration; such a configuration is more energetically favorable due to the possibility of steric interactions in a cis configuration.
Usually, free rotation should be able to take place about a single bond between a carbonyl carbon and amide nitrogen, the structure of a peptide bond. However, the nitrogen in this case has a lone pair of electrons. These electrons are near a carbon-oxygen bond. As a result, a reasonable resonance structure can be drawn, in which a double bond links the carbon and nitrogen. Consequently, the oxygen has a negative charge and the nitrogen has a positive charge. Rotation around the peptide bond is therefore inhibited by the resonance structure. Additionally, the real structure is a weighted hybrid of these two structures. The resonance structure is a significant factor in depicting the true electron distribution: the peptide bond has approximately 40% double-bond character. As a result, it is rigid.
Charges result in the peptide bond having a permanent dipole. The oxygen has a -0.28 charge, and the nitrogen has a +0.28 charge as a result of the resonance.
Contact: Sophia Wang
Phone:
E-mail: info@mc-biotech.com
Add: 2nd Floor,ECNU Science Park,No.1006 Jinshajiang Road, Putuo District, Shanghai